Library preparation from the enriched methylated DNA fraction (MBDE DNA samples)
Using the Zymo Pico Methyl Seq Kit on the MBD enriched Stylophora shallow and mesophotic samples from the pH experiment. For this library prep I also used the E. coli Non-Methylated Genomic DNA which is not supplied with the Zymo Methyl kit.
For this Library prep I used the protocol of meschedl with some adjustments.
Dilution of MBDE samples
I diluted the MBDE DNA samples to get a concentration of 0.5 ng/µl per each. Prepared new 0.5 tubes with:
| Sample | volume DNA | volume ultra pure water |
| S1 | 1µl | 24.2µl |
| S2 | 1µl | 4.4µl |
| S3 | 1µl | 0.4µl |
| S4 | 1µl | 12.6µl |
| S5 | 1µl | 6µl |
| S6 | 1µl | 5.4µl |
| S7 | 1µl | 4.4µl |
| S8 | 1µl | 2.8µl |
| S9 | 1µl | 4µl |
| S10 | 1µl | 4µl |
| S11 | 1µl | 0.4µl |
| S12 | 1µl | 4.2µl |
| S13 | 1µl | 2.4µl |
| S14 | 1µl | 28.6µl |
| S15 | 1µl | 1.8µl |
| S16 | 1µl | 9.8µl |
| S17 | 1µl | 1.8µl |
| S18 | 1µl | 95.2µl |
Dilution of Non-methlated eColi DNA
Need to dilute the 2.5ng/µl E. coli Non-Methylated Genomic DNA (spike-in) to be 0.025ng/µl, so a 1:100 dilution:
1µl E. coli non-methylated DNA
99µl ultra pure water
Sample Prep
Samples need to all include 1ng of sample, .05ng spike-in, and water up to 20µl
| sample | volume DNA | volume diluted spike | volume ultra pure water to 20µl |
| S1 | 2µl | 2µl | 16µl |
| S2 | 2µl | 2µl | 16µl |
| S3 | 2µl | 2µl | 16µl |
| S4 | 2µl | 2µl | 16µl |
| S5 | 2µl | 2µl | 16µl |
| S6 | 2µl | 2µl | 16µl |
| S7 | 2µl | 2µl | 16µl |
| S8 | 2µl | 2µl | 16µl |
| S9 | 2µl | 2µl | 16µl |
| S10 | 2µl | 2µl | 16µl |
| S11 | 2µl | 2µl | 16µl |
| S12 | 2µl | 2µl | 16µl |
| S13 | 2µl | 2µl | 16µl |
| S14 | 2µl | 2µl | 16µl |
| S15 | 2µl | 2µl | 16µl |
| S16 | 2µl | 2µl | 16µl |
| S17 | 2µl | 2µl | 16µl |
| S18 | 2µl | 2µl | 16µl |
Bisulfite Conversion
- Added 130µl lightning conversion reagent (from the kit) to each sample in a PCR tube
- Put tubes in the thermocycler Pico bisulfite conversion program: for this program I used the small thermocycler that we have in the lab (the SimpliAmp), I created the program inside the folder “methylation”.
These are the run settings:

Cleanup with columns
- Made 12 spin column, one for each sample
- Added 600µl M-binding buffer each to 12 spin columns
- Added 150µl of the bisulfite conversion reaction (all) to each individual tube
- Invert columns to mix
- Centrifuged columns at 12,000 rcf for 30 seconds and discarded flowthrough
- Added 100µl M-Wash buffer to each column
- Centrifuged columns at 12,000 rcf for 30 seconds and discarded flowthrough
- Added 200µl L-desulfonation buffer to each column
- Let them sit for 15 minutes
- Centrifuged columns at 12,000 rcf for 30 seconds and discarded flowthrough
- Added 200µl M-wash buffer to each column
- Centrifuged columns at 12,000 rcf for 30 seconds and discarded flowthrough
- Added 200µl M-wash buffer to each column
- Centrifuged columns at 12,000 rcf for 1 minute and 30 seconds and discarded flowthrough
- Added 8µl warmed (56C) DNA elution buffer to each column and let sit for 1 minute in new 1.5mL tubes to collect
- Centrifuged columns at 12,000 rcf for 30 seconds
Amplification with PrepAmp Primers
- Made Priming master mix on ice:
- 2µl 5X PrepAmp buffer * 13 = 26µl
- 1µl PrepAmp Primers (40µM) * 13 = 13µl
- Made new PCR tubes with 3µl of Priming master mix and 7µl of bisulfite treated DNA
- Kept those on ice
- Made PrepAmp Mix on ice:
- 1µl 5X PrepAmp buffer * 13 = 13µl
- 3.75µl PrepAmp PreMix * 13 = 48.75µl
- 0.3µl PrepAmp polymerase * 13 = 3.9µl
- For this amplification I used the big thermocycler that we have in the lab (the Bio Rad, not the SumpliAmp), I created the program inside the folder “methylation”, it is the second program inside the folder.

Note: During Step 3 of Cycle 1, add 5.05 μl PrepAmp mix. During Step 3 of Cycle 2, add 0.3 μl PrepAmp Polymerase.
- Run steps:
- 98 for 2 minutes
- 8 degrees for 1 minute
-
8 degree hold
Cycle 1: during hold vortex, spin tubes down, add 5.05µl PrepAmp Mix to each tube, vortex, spin down, and place back in thermocycler.
Cycle 2: during hold, vortex, spin tubes down, add 0.3µl PrepAmp Polymerase to each tube, vortex, spin down, and place back into thermocycler.
- 8 degrees for 4 minutes
- 16 degrees for 1 minute with 3% ramp rate
- 22 degrees for 1 minute with 3% ramp rate
- 28 degrees for 1 minute with 3% ramp rate
- 36 degrees for 1 minute with 3% ramp rate
- 36.5 degrees for 1 minute with 3% ramp rate
- 37 degrees for 8 minutes
- repeat back from the first step one time through again
Cleanup with Columns
- Made a 1.5mL tube for each sample, added 7:1 ratio DNA binding buffer, so 107.45µl of DNA binding buffer
- Put elution buffer in thermomixer 56 degrees
- Added DNA sample (15.35µl) to the appropriate 1.5mL tube
- Vortexed, spun down, and added to the column
- Centrifuged 12,000 rcf 30 seconds, discarded flowthrough
- Added 200µl DNA wash buffer to each column
- Centrifuged 12,000 rcf 30 seconds, discarded flowthrough
- Added 200µl DNA wash buffer to each column
- Centrifuged 12,000 rcf for 1 minute and 30 seconds, discarded flowthrough
- Transferred columns to 1.5mL tubes
- Added 12µl warmed elution buffer to each column directly
- Incubated 1 minute
- Centrifuged 12,000 rcf 30 seconds
First Amplification
- Made 1st Amp master mix:
- 12.5µl 2X Library Amp Mix * 13 = 162.5µl
- 1µl Library Amp Primer(10µM) * 13 = 13µl
- Added 13.5µl of the mix to new PCR tubes
- Added 11.5µl of cleaned and concentrated DNA sample to the appropriate new PCR tube
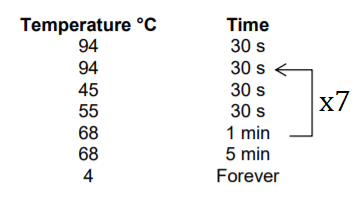
- Vortexed, spun down, and placed in thermocycler program 1st Pico Methyl Amp program. For this amplification I used the big thermocycler that we have in the lab (the Bio Rad, not the SumpliAmp), I created the program inside the folder “methylation”, it is the third program inside the folder.
These are the run settings (8 cycles total):
Cleanup with columns
- Made a 1.5mL tube for each sample, added 7:1 ratio DNA binding buffer, so 175µl of DNA binding buffer
- Put elution buffer in thermomixer 56 degrees
- Added DNA sample (25µl) to the appropriate 1.5mL tube
- Vortexed, spun down, and added to the column
- Centrifuged 12,000 rcf 30 seconds, discarded flowthrough
- Added 200µl DNA wash buffer to each column
- Centrifuged 12,000 rcf 30 seconds, discarded flowthrough
- Added 200µl DNA wash buffer to each column
- Centrifuged 12,000 rcf 1 minute and 30 seconds, discarded flowthrough
- Transferred columns to 1.5mL tubes
- Added 12.5µl warmed elution buffer to each column directly
- Incubated 1 minute
- Centrifuged 12,000 rcf 30 seconds
Amplification with Index Primers
- In PCR tubes combined the following:
| sample | volume DNA | volume LibAmp MM | volume i5 primer | volume i7 primer |
| S1 | 12µl | 11µl | 1µl 1 | 1µl 1 |
| S2 | 12µl | 11µl | 1µl 2 | 1µl 2 |
| S3 | 12µl | 11µl | 1µl 3 | 1µl 3 |
| S4 | 12µl | 11µl | 1µl 4 | 1µl 4 |
| S5 | 12µl | 11µl | 1µl 5 | 1µl 5 |
| S6 | 12µl | 11µl | 1µl 6 | 1µl 6 |
| S7 | 12µl | 11µl | 1µl 7 | 1µl 7 |
| S8 | 12µl | 11µl | 1µl 8 | 1µl 8 |
| S9 | 12µl | 11µl | 11µl 9 | 1µl 9 |
| S10 | 12µl | 11µl | 1µl 10 | 1µl 10 |
| S11 | 12µl | 11µl | 1µl 11 | 1µl 11 |
| S12 | 12µl | 11µl | 1µl 12 | 1µl 12 |
| S13 | 12µl | 11µl | 1µl 13 | 1µl 13 |
| S14 | 12µl | 11µl | 1µl 14 | 1µl 14 |
| S15 | 12µl | 11µl | 1µl 15 | 1µl 15 |
| S16 | 12µl | 11µl | 1µl 16 | 1µl 16 |
| S17 | 12µl | 11µl | 1µl 17 | 1µl 17 |
| S18 | 12µl | 11µl | 1µl 18 | 1µl 18 |
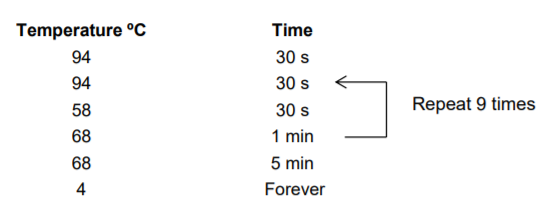
- Vortexed, spun down, and placed in thermocycler program 2nd Pico Methyl Amp program. For this amplification I used the big thermocycler that we have in the lab (the Bio Rad, not the SumpliAmp), I created the program inside the folder “methylation”, it is the fourth program inside the folder.
These are the run settings:
Cleanup with columns
- Made a 1.5mL tube for each sample, added 7:1 ratio DNA binding buffer, so 175µl of DNA binding buffer
- Put elution buffer in thermomixer 56 degrees
- Added DNA sample (25µl) to the appropriate 1.5mL tube
- Vortexed, spun down, and added to the column
- Centrifuged 12,000 rcf 30 seconds, discarded flowthrough
- Added 200µl DNA wash buffer to each column
- Centrifuged 12,000 rcf 30 seconds, discarded flowthrough
- Added 200µl DNA wash buffer to each column
- Centrifuged 12,000 rcf 1 minute and 30 seconds, discarded flowthrough
- Transferred columns to 1.5mL tubes
- Added 12µl warmed elution buffer to each column directly
- Incubated 1 minute
- Centrifuged 12,000 rcf 30 seconds
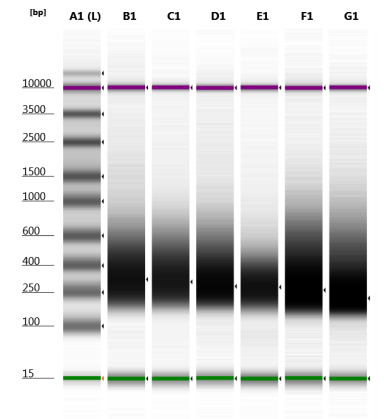
- Check DNA concentration with Nanodrop and quality with TapeStation (D5000)
Example of TapeStation results: